
Zirconium oxide (ZrO2) occurs in three modifications. The monoclinic form with a density of 5.6g/cm3, occurring in nature as baddeleyite (zirconia), is stable up to 1000°C. Between 1000°C and 1150°C ZrO2 forms a tetragonal modification with a density of 6.1g/cm3, which is transferred to a cubic form above 2350°C having a density of 6.27g/cm3 and a melting point of 2715°C. The significant differences of the densities of tetragonal and monoclinic ZrO2 may lead to stress in workpieces, which may lead to cracks upon heating and cooling caused by the changing of modifications.
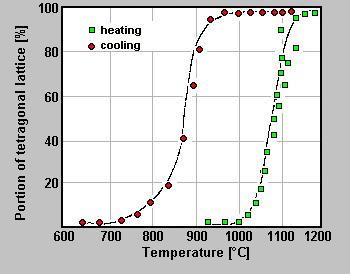
Monoclinic-tetragonal transformation of pure ZrO2 during heating and cooling within the area of the transformation temperature showing a hysteresis on heating and cooling cycles (from: Introduction to Zirconia, MEL, 1986)
Therefore the cubic form should be stabilized for technical ceramics. This is done via the addition of CaO, MgO and Y2O3, which form cubic solid solutions with ZrO2. Chemical data of unstabilized zirconium dioxide as well as CaO-stabilized zirconium dioxide , MgO-stabilized zirconium dioxide and Y2O3-stabilized zirconium dioxide are given on separate pages. By using these additions the so formed stabilized zirconias are no longer subjected to any changing of modifications. So-called partly stabilized ZrO2 are stable against temperature shocks by formation of microcracks; in similar way such mircocracks are used directly to increase the strenght of Al2O3-ceramics. Since ZrO2 is not wetted by many metallic melts, it can be used for crucibles, troughs, filters, coatings and similar applications. Monoclinic zirconia is the basic material of the product Zirconia-Coating ZR-M. CaO-stabilized zirconia is used in the products Zirconia-Coating ZR-A and Zirconia-Coating ZR-O.
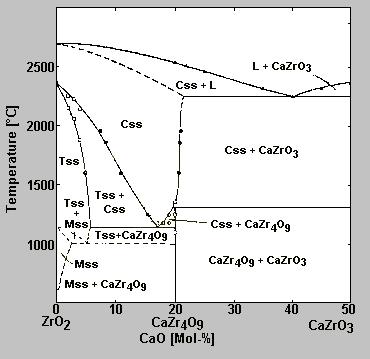
Phase diagram of the system ZrO2 -CaO. (from: Stubican V. S. und J. R. Hellmann: Phase Equilibria in some Zirconia Systems, p.25; Advances in Ceramics, Vol. 3, Science and Technology of Zirconia, 1981)
Properties of yttrium oxide |
|
Formular |
ZrO2 |
Molecular mass |
123.22 g/mol |
Density (monoclinic) |
5.6 g/cm³ |
Density (tretragonal) |
6.1 g/cm³ |
Density (cubic) |
6.27 g/cm³ |
Melting point |
2700°C (4892°F) |
CAS-No. |
1314-23-4 |
EINECS-No. |
215-227-2 |
© 2023 Büro für angewandte Mineralogie · Dr. Stephan Rudolph · D-47918 Tönisvorst
These recommendations are believed to be correct. However, no guarantee of their accuracy is given. Therefore, purchasers shall make their own tests to determine suitability for their use. These products are offered for industrial and related uses (e.g. research and development) only. However the user must take the necessary precautions appropriate for products containing chemicals. This description does not imply the absence of any patents, the responsibility whatsoever solely rests with the user.
www.a-m.de