
Hexagonal boron nitride (α-BN) is a white, talclike synthetic ceramic material with outstanding properties. It is a high-temperature solid lubricant, good dielectric, good thermal conductor and shows high electrical resistivity. Furthermore, it is chemically inert and not wetted by many metal melts as well as silicate and salt melts. Boron nitride is stable in air up to 1000°C, under vacuum up to 1400°C and can be used in argon gas atmosphere up to 2200°C and N2 up to 2400°C.
Boron nitride was first prepared in 1842 by reacting boric acid with potassium cyanide. Commercial production was launched in the 1950s by Carborundum and Union Carbide. Today the following synthesis routes are used for commercial production:
(1) Reaction of boric oxide with melamine according to
3B2O3 + C3H6N6 → 6BN + 3CO2 + H2O (“melamine-process”)
(2) Reaction of boric oxide with NH3 by using tri-calcium phosphate as a carrier according to
B2O3 + NH3 → BN + H2O (“trical-process”)
(3) Exothermic reaction of boric oxide with magnesium in presence of N2 according to
B2O3 + 3Mg + N2 → 2BN + 3MgO (“SHS-process”, self-propagating high-temperature synthesis)
The resulting boron nitride is well crystallized in the hexagonal structure showing platelets with a dimension of 1-10µm and a thickness of 0.1 - 0.5µm. The crystallinity however is influenced by the temperature the synthesis takes place.
.
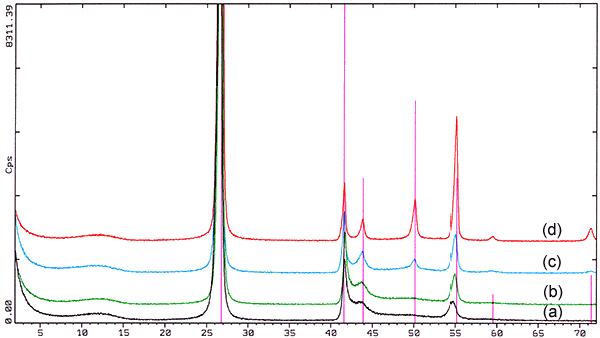
Fig. 1. X-ray diffraction (2Θ-Scale, radiation CuKα=1.5406Å) of boron nitride powders produced by the melamine-process showing various degrees of crystallinity caused by different reaction temperatures during synthesis from (a) low crystallinity produced at lower temperature to (d) high crystallinity produced at higher temperature.
Boron nitride is available as a powder with BN concentrations as low as 92% for refractory purposes to >98.5% for ceramic applications. High purity grades are >99.5%.
Companies involved in the production of BN include Advanced Ceramic Corp. (formerly Union Carbide) and Saint-Gobain Advanced Ceramics Corp. Boron Nitride (formerly Carborundum Corp. Boron Nitride) in the United States; Sintec Keramik UK (formerly Boride Ceramics & Composites and owned by Sintec Keramik GmbH & Co. KG, Germany) in the United Kingdom; SHS Cerámicas, Spain; Wacker Ceramics (formerly Elektroschmelzwerk Kempten) and H. C. Starck GmbH & Co. KG in Germany; and Denki Kagaku Kogyo, Kawasaki Steel Corp., Shin-Etsu Chemical Co. Ltd. and Showa Denko KK in Japan.
Prices for hexagonal BN range from ~$80-120/kg for standard qualities depending on purity of BN, and may reach prices up to $200-400/kg for high-purity and tailor-made grades. Because some producers increased their production during the last years in order to overcome the shortages of the past years prices are not expected to rise considerably in the near future.
Boron nitride shapes are produced by hot isostatic pressing of BN powder by using calcium borate glass as a binder. Large quantities of BN are used for the production of engineering ceramics like brake rings for horizontal continuous steel casting and TiB2/BN composites used as evaporator boats for vacuum metallization.
Other applications include those in the field of cosmetics, as catalysts and as a starting material for the production of cubic BN. Due to its extreme hardness, second to diamond, cubic β-BN is used as a hard material.
The good non-wetting behavior as well as the good high-temperature lubrication make BN an excellent release and parting agent for the production of aluminum and magnesium castings as well as glass forming and superplastic forming of titanium sheets for aerospace applications. In these applications boron nitride is used as a dispersion in a carrier (water or alcohol) blended with refractory binders, that are applied like wall paint. Such coatings are produced by Advanced Ceramic Corp., Saint-Gobain Advanced Ceramics Corp. Boron Nitride and ZYP Coatings Inc. in the United States and by Wacker Ceramics and Büro für angewandte Mineralogie in Germany.
Stephan Rudolph, Büro für angewandte Mineralogie
from: American Ceramic Society Bulletin, 81 (2001) 8, pp. 34-35
![]() Gesamte Veröffentlichung als pdf herunterladen.
Gesamte Veröffentlichung als pdf herunterladen.
© 2023 Büro für angewandte Mineralogie · Dr. Stephan Rudolph · D-47918 Tönisvorst
Vorstehende Angaben entsprechen den im Labor und Betrieb gemachten Erfahrungen. Sie können jedoch in Anbetracht der wechselnden Verhältnisse nur als Anhalt dienen und sind in diesem Sinne als unverbindlich anzusehen. Diese Produkte sind nur für den industriellen Bereich und vergleichbare Anwendungen (z. B. Forschung und Entwicklung) bestimmt. Die beim Umgang mit Chemikalien üblichen Vorsichtsmaßregeln sind zu berücksichtigen. Schutzrechte Dritter bitten wir zu beachten.
www.a-m.de