|
|
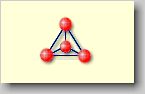 |
Nesosilicates
[neso (gr.) = island] or independent tetrahedral silicates:
The isolated [SiO4]4--tetrahedrons exist as independent
Units wihich are linked together by cations like Fe2+, Mg2+
or Zr4+. Examples for nesosilicates are olivine (Fe,Mg)2[SiO4],
zircon Zr[SiO4], but also the minerals of the garnet group like
almandine Fe2+3Al2[SiO4]. |
|
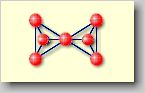 |
Sorosilicates
[soro (gr.) = group] or double tetrahedral silicates: Two
[SiO4]4--tetrahedrons are linked together by sharing
on oxygen atom forming a [Si2O7]6--ion.
This structure is realized in e.g. thortveitite Sc2[Si2O7]
. |
|
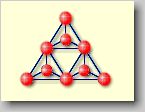 |
Cyclosilicates
[cyclo (gr.) = ring] or ring silicates: Three [SiO4]4--tetrahedrons
are linked together forming a ring with the formula [Si3O9]6-
as in benitonite BaTi[Si3O9]. A further variation
is possible by forming a ring consisting of six [SiO4]4--tetrahedrons
as an [Si6O18]12--ion. The beryl with
the chemical formula Al2Be3[Si6O18]
can be named as an example. |
|
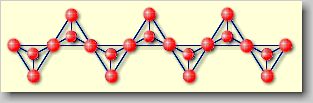
| Inosilicates
[ino (gr.) = thread] or chain silicates: Chain silicates
are realized by linking [SiO4]4--tetrahedrons in
a way to form continous chains. They may be represented by a composition
of [SiO3]2-. A typical example is diopside
CaMg[Si2O6], in which the "endless" chains als hold
together by Ca2+- and Mg2+-ions. |
 |
|
| Double
chain silicates: Two silicate chains of the ionsilicates are linked
by the corners forming double chains yielding [Si4O11]6-
-ions as realized in glaucophane Na2Mg3Al2[(OH,F)|Si4O11]2.
Double chain silicates are commonly grouped with the inosilicates. |
 |
|
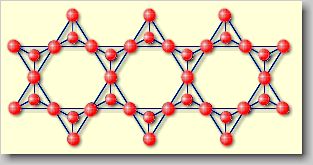
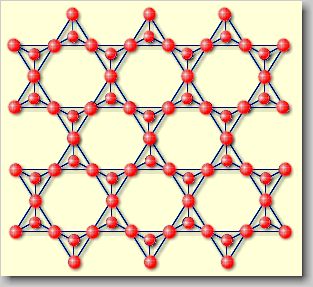
| Phyllosilicates
[phyllo (gr.) = sheet] or sheet silicates: They are formed
if the above described [SiO3]2-chains are linked
together to form continuous sheets with the chemical formula [Si2O5]2-.
Sheet silicates are for example pyrophyllite Al2(OH)2[Si4O10]
or talc Mg3(OH)2[Si4O10]. |
 |
|
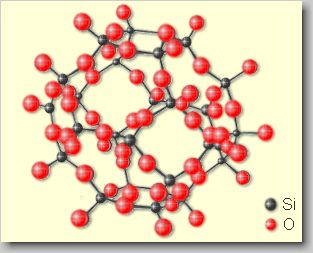
| Tectosilicates
[tecto (gr.) = framework] or framework silicates: Tectosilicates
are formed by a [SiO4]4--tetrahedron, which is linked
together with four tetrahedrons in a three-dimensional framework in such
a way, that the tetraherdons share one oxygen atom. This yield a ratio
of (Si,Al):O=1:2, where silicon may be replaced by aluminium. Tectosilicates
are among others feldspars like orthoclase K[AlSi3O8]
as well as zeolites like natrolite Na2[Al2Si3O10]·2H2O. |
 |