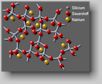
Fig. 1 Model structure of glass
|
Glasses
- contrary to crystalline materials - show no regular arrangement of
their
atoms. For example, the SiO4-tetrahedrons of silicate
glasses
are irregulary interlaced (cf. Fig. 1).
The
relatively large gaps can be filled by cations like sodium or potassium.
In order to differentiate glasses from crystalline materials, the changing of volume is described when the respective substances are cooled from melting temperature to ambient temperature (cf. Fig. 2). Here such systems are compared which (a) solidify by crystallization and (b) by formation of glass. One detects that crystalline materials go through a enormous variation in volume with the melting temperature TS, they crystallize (red line). In contrast to crystalline materials glasses condense over a wide temperature range (blue curve) also beyond TS. In the area of the transformation temperature Tg they change from the plastic state to the brittle state when cooled slowly (blue dotted line). Rapid cooling leads to an undercooled liquid (blue line). The changing of viscosity described here is fundamental for the for the processing of glasses and depends only on the chemical composition of the respective glasses. |